Ensuring the quality, purity, and identity of raw materials is a critical requirement in every pharmaceutical, food, chemical, and research laboratory. Among various analytical tools available for material identification, FT-IR (Fourier Transform Infrared) Spectroscopy stands out as one of the most reliable, fast, and non-destructive techniques.
This blog post explores how FT-IR spectrometers are used to analyze and confirm raw materials, focusing specifically on three commonly used substances:
- Sodium Bicarbonate
- Sodium Chloride
- Microcrystalline Cellulose (MCC)
We will cover the principles of FT-IR, sample preparation using KBr pellets or ATR, international standards, and the limitations and strengths of the method for each raw material.


Introduction: Why FT-IR Spectroscopy for Raw Material Testing?
In any QC (Quality Control) laboratory—especially in pharmaceutical and nutraceutical manufacturing—raw material identification is a mandatory step before approval for production. Instruments like FT-IR spectrometers allow rapid and accurate verification of the chemical identity of incoming materials.
Why FT-IR for Raw Material Identification?
- ✔ Fast and non-destructive
- ✔ Requires minimal sample preparation
- ✔ Produces highly specific “fingerprint” spectra
- ✔ Widely accepted by international pharmacopeias (USP, BP, Ph. Eur, IP)
- ✔ Sensitive to chemical structure, functional groups, and molecular interactions
These advantages make FT-IR an essential tool in compliance with GMP, GLP, and ISO 17025 laboratory standards.
Understanding FT-IR Spectroscopy in Simple Terms
Fourier Transform Infrared Spectroscopy is based on the principle that molecules absorb infrared radiation at specific frequencies related to their chemical bonds. When a sample is exposed to IR light, each type of chemical bond vibrates in a unique manner.
These vibrations create a spectrum—a graphical representation of absorbance vs. wavenumber (cm⁻¹)—that acts like a chemical fingerprint.
Key Regions in an FT-IR Spectrum
- 4000–2500 cm⁻¹: Functional group region
- 2500–1500 cm⁻¹: Multiple bond region
- 1500–400 cm⁻¹: Fingerprint region (most specific for identification)
By comparing a test spectrum with a reference (standard) spectrum, analysts can confirm the material’s identity.
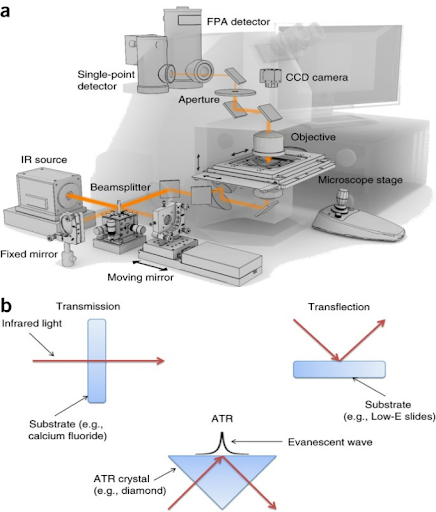
Sample Preparation Techniques in FT-IR Analysis
FT-IR samples can be measured in different ways. For raw materials like the ones we are discussing, the two most common sample preparation methods are:


KBr Pellet Method
This is a classical method widely recognized by pharmacopeial bodies.
Procedure Overview
- Mix ~1–2 mg of sample with ~100 mg of dry potassium bromide (KBr).
- Grind the mixture into a fine, homogeneous powder.
- Compress into a transparent pellet using a hydraulic press.
- Place pellet in the FT-IR holder and record the spectrum.
Advantages
- High-quality spectra with strong signal
- Suitable for regulatory (pharmacopeial) compliance
- Ideal for raw materials and solid powders
Limitations
- Sensitive to moisture (KBr absorbs water)
- Time-consuming compared to ATR
- Not ideal for hygroscopic materials unless dried

ATR (Attenuated Total Reflectance) Method
More modern and user-friendly.
Procedure Overview
- Place sample directly on the ATR crystal.
- Apply pressure.
- Collect spectrum—no pellet required.
Advantages
- Rapid testing
- Minimal preparation
- Hygienic and less chance of contamination
Limitations
- Slightly lower resolution in some cases compared to KBr
- Not accepted in certain monographs unless validated
Both methods are acceptable provided the laboratory uses validated procedures and meets regulatory guidelines.
International Standards and Guidelines for FT-IR Raw Material Testing
Quality Control laboratories must follow global pharmacopeial and regulatory standards for FT-IR testing:
✓ USP (United States Pharmacopeia)
- <197> Spectroscopic Identification Tests
- <843> Infrared Spectroscopy
- <1058> Analytical Instrument Qualification
✓ European Pharmacopoeia (Ph. Eur.)
- 2.2.24 IR Spectrophotometry
✓ British Pharmacopoeia (BP)
- IR identification under individual monographs
✓ Indian Pharmacopoeia (IP)
- IR absorption tests for salts and polymers
✓ Quality Standards
- ICH Q7 (GMP for APIs)
- ICH Q2 (Method Validation)
- ISO/IEC 17025 (Laboratory Competence)
- FDA 21 CFR 211 (Laboratory Controls)
These references ensure FT-IR results are accurate, reliable, and legally compliant.
FT-IR Analysis of Sodium Bicarbonate
About Sodium Bicarbonate
Sodium bicarbonate (NaHCO₃) is widely used in pharmaceuticals, food, and industrial applications. As a carbonate salt, it contains the bicarbonate ion, which shows characteristic IR absorption.
Why FT-IR Works for Sodium Bicarbonate
NaHCO₃ contains:
- C–O
- C=O
- O–H
functional groups that strongly absorb IR radiation
Major FT-IR Peaks of Sodium Bicarbonate
| Wavenumber (cm⁻¹) | Interpretation |
|---|---|
| ~1450–1500 | CO₃ asymmetric stretching |
| ~1010–1050 | C–O stretching |
| ~880 | Bicarbonate bending |
| ~3400 | O–H stretch due to moisture or bicarbonate |
These peaks form a specific pattern used for identification.
Acceptance Criteria
A test sample passes identity testing if:
- All major peaks match the reference spectrum
- The fingerprint region (900–600 cm⁻¹) aligns with standard
- No unexpected peaks appear
FT-IR is one of the most reliable and preferred methods for confirming sodium bicarbonate identity.

FT-IR Analysis of Sodium Chloride
About Sodium Chloride
Sodium chloride (NaCl) is among the simplest and most common inorganic materials used in industries. It is a fully ionic compound with a stable crystal structure.
Why FT-IR Does Not Work for Sodium Chloride
Pure NaCl:
- has no IR-active vibrational modes
- does not absorb IR light
This is because IR absorption requires changes in dipole moment during molecular vibrations—something ionic crystal lattices like NaCl do not exhibit.
Important Note
NaCl is so IR-transparent that it is often used to manufacture:
- Infrared window plates
- Cells
- Beam splitters
Alternative Identification Methods
Since FT-IR cannot identify NaCl, pharmacopeias recommend:
- Silver nitrate test (white precipitate of AgCl)
- Flame test for sodium
- Conductivity test
- Assay via titration
Therefore, FT-IR cannot measure or identify pure sodium chloride, but it can detect impurities if organic contaminants are present.
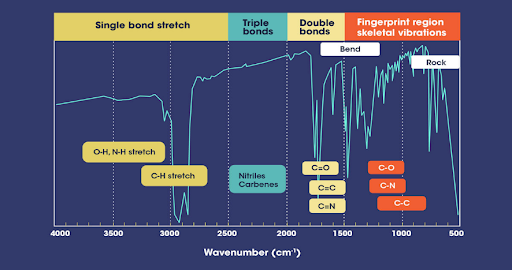
FT-IR Analysis of Microcrystalline Cellulose (MCC)
About MCC
Microcrystalline Cellulose is a purified, partially depolymerized form of cellulose used as:
- Diluent
- Binder
- Disintegrant
in tablets and capsules.
As an organic polymer, it has a complex structure that produces a rich infrared spectrum.
Why FT-IR Works for MCC
MCC contains:
- O–H groups
- C–H bonds
- C–O–C glycosidic linkages
which all show strong IR absorption.
Major FT-IR Peaks of MCC
| Wavenumber (cm⁻¹) | Interpretation |
|---|---|
| 3300–3500 | O–H stretching (broad) |
| 2890–2950 | C–H stretching |
| 1420–1430 | CH₂ bending |
| 1360–1375 | C–H symmetric bending |
| 1020–1100 | C–O–C stretching (key fingerprint) |
| 900–910 | β-glycosidic deformation |
Reasons for High Specificity
The fingerprint region (1500–400 cm⁻¹) contains intricate peaks unique to cellulose derivatives, making FT-IR ideal for identifying MCC grades and detecting adulteration.
Acceptance Criteria
- Peaks must match pharmacopoeial reference spectrum
- No additional peaks should indicate foreign organic matter
- Moisture-related peaks should remain within expected range
Thus, FT-IR serves as an essential tool for MCC verification.
Comparison Table: FT-IR Suitability for the Three Raw Materials
| Raw Material | FT-IR Identification | Why? |
|---|---|---|
| Sodium Bicarbonate | ✔ Yes | Contains IR-active functional groups |
| Sodium Chloride | ❌ No | No IR-active vibrations (ionic) |
| Microcrystalline Cellulose | ✔ Yes | Complex organic polymer with many IR-active groups |

Practical Tips for QC Analysts Using KBr FT-IR
- Keep KBr DryMoisture drastically affects the pellet quality and spectrum.
- Use Correct Ratios1–2 mg sample for 100 mg KBr gives the best results.
- Clean Mortar and PestleAny leftover material can contaminate the sample.
- Validate the MethodFollow ICH Q2 guidelines.
- Check System SuitabilityUsing a polystyrene film to verify wavenumber accuracy is required by pharmacopeias.
- Document EverythingClear traceability ensures GMP compliance.
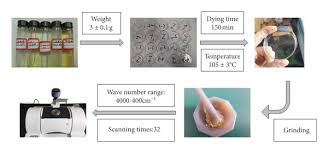
Common Mistakes and How to Avoid Them
Mistake 1: Using moist KBr
→ Causes broad O–H peaks and invalid spectra.
Solution: Dry KBr at 105°C before use.
Mistake 2: Applying too much sample
→ Leads to saturated peaks.
Solution: Use micro-quantities.
Mistake 3: Dirty ATR crystal
→ Contaminates spectra.
Solution: Clean with ethanol before and after each run.
Mistake 4: Incorrect pellet pressure
→ Results in cloudy pellets.
Solution: Apply consistent pressure.
Conclusion
FT-IR spectroscopy is one of the most versatile, rapid, and reliable techniques for identifying a wide range of raw materials. For compounds like Sodium Bicarbonate and Microcrystalline Cellulose, FT-IR provides clear, unique spectra that can be directly matched with pharmacopeial references for identity confirmation.
However, it’s equally important to understand the limitations of FT-IR. For Sodium Chloride, FT-IR cannot be used because NaCl lacks IR-active chemical bonds. QC laboratories must therefore use alternative wet chemical tests as required by pharmacopeial monographs.
The combination of FT-IR’s accuracy, speed, and minimal sample preparation makes it a cornerstone method in modern quality control environments. With proper calibration, validation, and adherence to international standards, FT-IR spectrometers ensure that raw material identity testing is both compliant and efficient.
25 FAQs on FT-IR Spectroscopy for Raw Material Identification
- What is FT-IR spectroscopy and why is it used in raw material testing?
FT-IR (Fourier Transform Infrared Spectroscopy) is an analytical technique used to identify materials based on their unique infrared absorption patterns. It is used in QC labs because it is fast, reliable, non-destructive, and accepted by all major pharmacopeias.
- How does FT-IR help in identifying raw materials like Sodium Bicarbonate and MCC?
These materials contain IR-active chemical bonds that absorb infrared radiation at specific frequencies. Their spectra serve as unique fingerprints for identification.
- Can Sodium Chloride be identified using FT-IR?
No. Sodium chloride (NaCl) does not have IR-active vibrations, so it does not produce a characteristic spectrum. Alternative chemical tests must be used.
- Why does Sodium Chloride fail to show peaks in FT-IR?
NaCl has a purely ionic lattice structure. There is no dipole moment change during vibrations, which is required for IR absorption.
- What international standards govern FT-IR testing in QC labs?
Key references include:
- USP <197>, <843>, <1058>
- Ph. Eur 2.2.24
- BP (Infrared Identification tests)
- IP (KBr pellet method)
- ICH Q2, Q7
- ISO/IEC 17025
- FDA 21 CFR Part 211

- What sample preparation methods are used in FT-IR for raw materials?
The two most common methods are:
- KBr pellet method
- ATR (Attenuated Total Reflectance)
- Which method is better—KBr pellets or ATR?
KBr pellets generally offer better resolution and are preferred when pharmacopeias explicitly require them. ATR is faster and more convenient but must be validated before routine use.
- How is a KBr pellet prepared for FT-IR testing?
A small amount of sample (1–2 mg) is mixed with dry KBr (100 mg), ground into fine powder, and compressed into a transparent pellet using a hydraulic press.
- Why must KBr be completely dry before preparing a pellet?
KBr is highly hygroscopic. Moisture introduces unwanted O–H bands in the spectrum, which can interfere with interpretation and cause failure of identification.
- What type of spectrum is expected for Sodium Bicarbonate?
A spectrum with strong absorption bands near ~1470 cm⁻¹ (CO₃ stretching), 1030 cm⁻¹ (C–O stretch), and 880 cm⁻¹ (bicarbonate bending).
- What type of spectrum is expected for Microcrystalline Cellulose (MCC)?
A complex organic spectrum with peaks at ~3340 cm⁻¹ (O–H stretching), ~2900 cm⁻¹ (C–H stretching), ~1430 cm⁻¹ (CH₂ bending), and strong C–O–C peaks in the 1100–1020 cm⁻¹ region.
- What factors can affect FT-IR spectral quality?
Moisture, sample particle size, pellet thickness, dirty ATR crystals, poor pressure, and baseline shifts can affect spectral accuracy.
- What is the fingerprint region in an FT-IR spectrum?
The region between 1500–400 cm⁻¹, where complex absorption patterns occur. It is highly specific and used for confirming identity.
- Is calibration of FT-IR instruments required?
Yes. Instruments must be calibrated regularly using standard materials such as polystyrene films, as required by USP <1058> and Ph. Eur.
- Can FT-IR detect impurities in raw materials?
While FT-IR is mainly used for identity testing, it can detect certain organic impurities. However, it is not typically used for quantitative impurity analysis.
- Can FT-IR quantify Sodium Bicarbonate, MCC, or Sodium Chloride?
No. FT-IR is primarily a qualitative technique for identity testing. Other analytical methods are required for assay and impurity quantification.
- How much sample is needed for FT-IR testing?
Typically 1–2 mg for KBr pellets and a few milligrams for ATR.
- What if the FT-IR spectrum fails to match the reference?
The material should be quarantined, and the test repeated. If mismatch persists, possible contamination or mix-up must be investigated according to GMP procedures.
- Do pharmacopeias require FT-IR identification for all three materials?
- Sodium Bicarbonate: Yes, FT-IR is part of identity test.
- Microcrystalline Cellulose: Yes, IR identification is mandatory.
- Sodium Chloride: No, IR is not used for identity.
- Is ATR acceptable in place of KBr pellets for pharmacopeial compliance?
Yes, but only if the method is validated and equivalence with KBr spectra is demonstrated.
- How long does it take to perform FT-IR identification?
ATR: 1–2 minutes
KBr pellet: 5–10 minutes depending on preparation
- Can FT-IR distinguish between different grades of MCC?
Yes. Variations in crystallinity and polymer chain length create subtle spectral differences that can be monitored.
- What GMP requirements apply to FT-IR raw material testing?
Proper documentation, qualified instruments, traceability, validated methods, and SOP-based testing are essential under GMP and FDA 21 CFR 211.
- What is the role of the reference standard in FT-IR analysis?
Reference spectra (typically pharmacopeial standards or certified reference materials) are used for comparing and confirming the identity of test samples.
- What is the biggest limitation of FT-IR in raw material analysis?
FT-IR cannot detect materials that lack IR-active vibrations (e.g., NaCl) and cannot quantify components with high accuracy—it is mainly an identity test.
Contact :
- Mobile/WhatsApp: +971526191767
- Email: sales@apex-instrument.com



